This article is part of the special season start. All parts:
- Cast off - how to get the new season off to a smooth start
- Checklist: Fit for the new season - the checklist
- Cleaning, polishing and care
- The best care and cleaning products for boats
- Last-minute tips from the experts at Pantaenius for the start of spring
- Checklist for the boat trailer
- The best tips from the Pantaenius specialist
- Changing the oil in a boat engine - here's how!
- Special pumps - changing oil in no time at all
- To check the cooling circuit
- How to renew the antifouling
- Boat equipment - the more, the better
The easiest way to remove coarse dirt from the entire boat is to clean it with a scrubbing brush, water hose and shampoo. Once the coarse dirt has been removed, the gelcoat and paintwork often still need to be polished. The skipper needs a polish for this.
It consists of a liquid containing small grains. The coarser the grains, the more the polish cleans and removes more colour material from the paint layer. So if you want to polish particularly matt surfaces, use a product with coarse grains. But be careful: too much of this so-called paint cleaner will quickly reduce the thickness of the coating. However, it is not only important to clean and shine surfaces, but also to seal them against new dirt. Some polishing agents already do this during the polishing process with Teflon or wax.

If, on the other hand, you work with a harsh paint cleaner, you usually have to re-seal with wax. These waxes are applied in the same way as the polish, using a polishing pad or disc. Owners of boats with a teak deck or bathing platform are often annoyed by the grey surface. True classic enthusiasts claim that this is the way it should be and only clean the teak with a scrubbing brush and water. Those who want to freshen up their wood, on the other hand, use teak cleaner, freshener and oil. However, a clean boat doesn't just include the large surfaces, but also the small details such as fittings, windows, rubbers and fenders.
Metal objects are polished with standard metal cleaners from the chemist or special metal care products from the boat shop. Glass panes are cleaned with "Glasrein", which can also be found on the shelves of drugstores or supermarkets. Special products for refreshing acrylic glass panes can be obtained from boat accessories. Note: Never clean acrylic glass panes with thinner or spirit, as these agents attack the surface.
Rubber care products that soften window and rubbing strip rubbers and protect them from the weather can be purchased from a boat shop. The same applies to fender cleaner. Some of these products can also be used as inflatable boat cleaners. There are special products for upholstery with artificial leather covers, as well as for the fabrics and carpets on board. For carpets and fabric upholstery, however, carpet foam from the DIY store is often sufficient. Particularly stubborn stains can form on the tarpaulin. These can be removed with so-called Schimmelex or Schimmefresser from a spray bottle. Subsequent impregnation is important, as mould eaters not only remove the mould, but also the protective layer.

Another dirty corner is the bilge. In this area, you not only have to deal with oily and stubborn dirt, but also with water. Special bilge cleaners dissolve the dirt and form an emulsion from the oil-water mixture, which can simply be wiped off with a cloth. Algae growth or yellowish discolouration on the hull can also be tackled with special agents such as "Algenol". Clean zips with a brush and treat them with silicone-based sprays, which also significantly improve smooth running.
Tip: Linen
Cordage is one of the most heavily used pieces of equipment on board. To ensure that the expensive ropes last longer, they also need to be cleaned regularly. In addition to a routine visual inspection, they should be washed once a year. This removes dirt particles from the synthetic fibres. Use a mild detergent for washing, Never use fabric softener. This can damage the fibres. Place the linen together in a textile bag so that they do not get tangled in the crevices of the drum. Only wash at 30 degrees on a gentle cycle, do not spin or machine dry. Hang the wet linen on a washing line in large bays to dry.

Polishing
The gentlest, but also most laborious polishing method is hand polishing with cotton wool or a cloth. This method is only used for light touch-ups and small areas. If you want to give the entire boat a high gloss, you need to use machines. Specialised polishing machines are available in two versions: The hobby version from the DIY store and the professional polisher from a specialist dealer. With the simple polishing machine, a disc swings back and forth. The polishing disc or bonnet is fixed to it with a tensioning strap or Velcro fastener. The same applies to the professional machine.
Your polishing disc rotates in a circle like an angle grinder. The difference to a conventional angle grinder is the speed control. This is because high speeds generate high temperatures between the polishing head and the surface. As a result, you quickly grind or burn marks into paint or plastic. However, it doesn't always have to be a special polishing machine. Polishing plates are also available for conventional DIY tools, such as drills with rubber discs, eccentric, delta and orbital sanders. Hobby craftsmen can also achieve acceptable results with these "polishing machines". The polishing discs for professional and hobby machines are available as lambskin or foam versions in DIY stores, car and boat accessory shops or specialist stores.

The situation is similar for metal parts on deck. "In pure, salt-free air, rust is of little concern to steel," writes Nigel Warren in his book "Corrosion on Yachts" (Delius Klasing), but also: "In salty air and correspondingly high humidity, the extent of corrosion is almost as severe as if the steel were lying in seawater." It is therefore important to also protect the metal parts on deck. This is because in humid air, for example during long voyages at sea, the electrons emitted are also absorbed by oxygen molecules dissolved in the water. Oxygen ions are formed, which immediately react with water molecules to form .
In practice: The surface must not be too warm during polishing, otherwise the liquid will dry too quickly and the product will not work properly. It is best to spread the polish over an area of about 0.50 m x 0.50 m and then work it with the machine. After a drying phase of a few minutes, wipe off the film with a clean cloth. To avoid streaks, work evenly when polishing or waxing.
Wo can I clean?
The boat can be polished both on land and in the water at the start of the season. However, water-based users must ensure that no polish drips into the water. This regulation applies nationwide. Cleaning boats with water is a different matter. This regulation (public use) is in the hands of the federal states. This means that each federal state has its own regulations for handling water.
You can find out which regulation applies to the respective federal state from the environmental authorities. The following applies to Hamburg and Schleswig-Holstein, for example: Water from the river, lake, sea and fresh water pipes is permitted for cleaning. The additional use of foam cleaners is generally not permitted on water moorings, but only on land with an appropriate water collector.

Corrosion
The reports from previous generations of cruisers make for adventurous reading: leaking decks and leaking oilskins, rusting fittings and shrouds. The wires had to be regularly rubbed with linseed oil to prevent corrosion. Today, ships are relatively maintenance-free, with GRP hulls only needing to be polished once a year. Metal parts are even less sensitive, as everything is now made of stainless steel. Stainless, mostly. The use of stainless steels began just over a hundred years ago, after it was discovered by chance that iron no longer rusts when it is alloyed with chromium.
Although the patent was not granted in Germany until 1918, the Germania shipyard used stainless steel for the first time in shipbuilding as early as 1908 for the construction of the "Germania". Since then, manufacturers have come up with different terms for stainless steel, such as V2A, V4A and Inox. However, even stainless steel is not completely corrosion-free. In long-term use in humid and salty environments, the German term "Nirosta" proves to be inappropriate; "Spätrosta" would be more appropriate. Because after a week on the Atlantic at the latest, it becomes clear: under certain circumstances, all steel corrodes. Ordinary steel (usually) becomes "stainless" through an alloy of iron, chromium and nickel. Steel becomes chromium-nickel steel, or simply: Stainless steel.
What is corrosion?
In principle, it is clear that corrosion or the colloquial term "rust" must involve moisture and that it is critical to join two dissimilar metals. But what are the connections? Rust is not corrosion. If rust can be seen on the surface of a metal, a chemical reaction has long since begun that transforms the metal. Rust is the result of corrosion.
A metal and moisture
If a metal part (e.g. shipbuilding steel) is immersed in an electrolyte solution (usually seawater), the metal atoms react by releasing electrons to form metal ions, which are transferred to the salt water solution: The metal dissolves.
The situation is similar for metal parts on deck. "In clean, salt-free air, rust is of little concern to steel," writes Nigel Warren in his book "Corrosion on Yachts" (Delius Klasing), but also: "In salty air and correspondingly high humidity, the extent of corrosion is almost as severe as if the steel were lying in seawater." It is therefore important to also protect the metal parts on deck. This is because in humid air, for example during long voyages at sea, the electrons emitted are also absorbed by oxygen molecules dissolved in the water. Oxygen ions are formed, which immediately react with water molecules to form

Hydroxide ions react, causing oxygen corrosion. Stainless steel is therefore susceptible to corrosion if it is exposed to an aggressive, moist and salty environment - or if its protective oxide layer is damaged during welding and drilling. It is therefore essential to treat it after processing - in the event of damage only to the passive layer during drilling or grinding, for example with a passivating agent (nitric or citric acid), electropolishing or pickling (see below). Polishing the surface actually strengthens the protective layer and makes it even harder.
Two metals interact with each other
Classic galvanic corrosion requires a less noble metal, a more noble metal, a connection between the two (permanently or temporarily electrically connected) and an electrolyte, i.e. an aqueous, electrically conductive connection between them.
An example: A stainless steel fitting is mounted on an aluminium pole without insulation, and the aluminium underneath slowly dissolves. In this case, the site of oxidation and the site of reduction lie on top of each other, so that one speaks of an electrochemical or galvanic element. The less noble aluminium, the so-called anode, is destroyed as oxidation takes place on the surface. The electrons flow through the aluminium to the surface of the stainless steel, where the reduction can take place unhindered. At the same time, the anode loses electrons, it suffers a dilution of its electron gas and thus loses its internal cohesion. The stainless steel thus becomes a local cathode. Metal atoms on the aluminium surface are converted into positive ions because the electrons are missing. The consequences for the material are devastating: the metal becomes brittle and flakes.

The current always flows from the anode (less noble metal) to the cathode (more noble metal), with the result that the less noble metal erodes or corrodes away over time. The less noble metal is attacked and at the same time protects the more noble metal by "sacrificing" itself. This is the reason why we attach sacrificial anodes to the metal parts of ships. A sacrificial anode made of magnesium, for example, protects the stainless steel on the propeller shaft from corrosion. In fresh water, the corrosion attack is reduced to a much smaller area. Because salt water is such a good conductor, the influence of corrosion in salt water spreads further, whereas the current in fresh water is greatly reduced with distance. A different metal should therefore also be used to protect against corrosion in fresh water.
Types of corrosion
Corrosion phenomena are manifold, and each has its own causes.
- Surface corrosion is a consistently uniform and erosive destruction of the entire surface of a metal and occurs, for example, on entire hull plates, but also on the surface of railing supports, shroud tensioners and other polished but otherwise unprotected stainless steel surfaces.
- Trough corrosion often occurs on steel plates on the underwater hull, for example under the paint or antifouling, if water has penetrated through cracks in the sealant. The diameter of the trough is usually far greater than the depth of the trough and the metal is generally removed unevenly.
- Pitting corrosionPitting corrosion, better known as pitting, is usually localised. In contrast to pitting corrosion, the depth of the (often needle-like) damaged areas is greater than the diameter of the hole, whereby individual holes can grow together. Pitting corrosion often occurs on the underwater hull of aluminium yachts (for example, if the wrong antifouling has been used or if there are ferrous contaminants on the material) as well as on rudder stocks and stainless steel cladding. It can also be caused by rust film.

- Crevice corrosion usually occurs in joints less than 0.5 millimetres in diameter, where water collects and remains for longer periods of time, for example under screw heads, because the chromium cannot combine with oxygen at this point and form an oxide layer. The more salt is dissolved in the water, the faster it progresses. Examples of this are shroud wires in the rollers, as well as under nuts and bolts.
- Contact corrosion occurs where two metals lie on top of each other and are connected by an electrolyte. The damage that occurs is similar to pitting corrosion. This can be remedied by a physical barrier between the metal parts, such as a plastic disc, Tefgel or Duralac.
It should therefore always be considered which type of fastening element is suitable for which fitting. Stainless steel is currently used almost exclusively, but this is not always the best solution. Fittings made of brass, gunmetal or bronze, for example, are most corrosion-resistant when fastened with brass screws. Sometimes it may still be necessary to fasten a fitting to the pole with a stainless steel rivet. This can work without any problems if the rivet is applied with sufficient tine. It is important that the fastening material is suitable for the surrounding water.
- Intergranular corrosion is caused by poorly executed weld seams. During welding, the oxide layer of the stainless steel is damaged and contaminated because chromium carbides escape next to the weld seam. If the weld is not cleaned and pickled, it is susceptible to corrosion. A pickling paste consisting of a reducing agent (hydrofluoric acid or hydrochloric acid) and an oxidising agent (nitric acid or hydrogen peroxide) removes the annealing colours and cleans the seams so that the metal can no longer continue to develop. At the same time, by releasing oxygen, it ensures that the new protective passive layer forms immediately.
- Condensation corrosion is a problem on poorly insulated steel ships, for example. Condensation forms on cold bridges between the outer panels and the insulating layer, which then runs down the ship's sides and cannot dry out due to a lack of ventilation, which in turn leads to corrosion.
- Selective corrosion is often found on brass seacocks that are not suitable for permanent use in salt water. The breakthrough made of simple brass with a golden yellow alloy of zinc and copper dezincifies over time in salty seawater. If zinc escapes according to the same principle, after the sacrificial anode described above also wears away from the material, what remains is a reddish, porous copper sponge, which has little mechanical strength due to its composition. The component can break even under low loads.
Corrosion under water
"It is often related to fasteners," writes Warren. "Brass screws and bolts are often the cause. But stainless steel also causes problems." In his opinion, joints between wood-wood and wood-GRP are particularly affected. According to Warren, a fastener with a corrosion-free alloy such as copper, bronze, gunmetal, cupronickel or nickel-chromium alloys should be used.
"Less resistant are stainless steel, brass and manganese bronze"; with the latter "dezincification will take place in water and breakage will occur". Warren also recommends: "Do not use stainless steel screws where they are not cathodically protected by a large base metal surface. Stainless steel screws are susceptible to pitting in water, especially in recesses." By this he means the threads under washers and screw heads; they are also highly susceptible to wood.

Professor Dieter Scharping puts it similarly in a text on stainless steel in yacht building: "Stainless steel components can (only) be used underwater without risk if they are surrounded by base metal surfaces, e.g. niroruders on steel or aluminium yachts. The probability of these parts being affected by pitting corrosion is lower than on a GRP boat."
Warren recommends the following fasteners underwater: aluminium or silicon bronze bolts for aluminium bronze seacocks, silicon or nickel aluminium bronze for gunmetal (stern tube), monel for stainless steel (rudder fittings) and stainless steel for aluminium (stern drive).
Galvanic damage
The most common causes of galvanic damage to the hull are different metals: lack of potential equalisation in the form of a sacrificial anode, insufficient use of sacrificial anodes or overprotection by an anode made of the wrong material; poor or incorrect protective coating; shore connection with a protective conductor connected to the on-board power supply and earthed to the sea valves/motor/keel bolts; strong current at the berth; berth in a harbour with many metal ships or even a sheet pile wall.
However, in addition to the galvanic damage caused by the corrosion system itself, the ship may also suffer electrolytic damage, for example due to insulation faults in the on-board electrical system, short circuits or other external influences. To protect the ship from electrolytic damage, the earthing should be connected to the shore power cable via the earth cable.
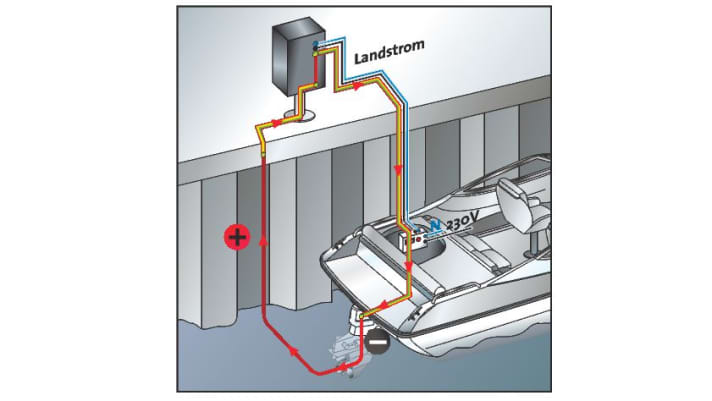
What can you do?
There is no permanent means of protection. However, corrosion can be minimised. There are four ways to do this:
- Oils, greases and water-displacing substances that can be brushed or sprayed on and that coat the workpiece with a film that prevents water from coming into contact with the metal surface. Disadvantages: After treating stainless steel parts with oil, they often appear greasy and unclean. Or the protective effect is worn away through use, e.g. when lines are being foiled on cleats or railing supports coated with Ballistol Seal.
- Agents that are added to water to reduce its corrosive effect. Example: Antifreeze in the internal circuit of the diesel engine.
- Agents that reduce humidity. These include, for example, silica or dehumidifier granules.
- A sacrificial anode or galvanisation that sacrifices itself to the more noble metal in order to protect it.

Rust removal and protection
It is important not to damage the passive layer of the stainless steel. If this happens, corrosion will soon form. Normally, the passive layer will rebuild itself. To support the rebuilding process, 80 per cent phosphoric acid also helps. The rust is converted into water-insoluble iron phosphate and the passive layer builds up again.
Oxalic acid is a household remedy for rusty stainless steel parts or traces of rust that have run off the gelcoat. Mix with warm water, about 100 grams per bucket. Mix, apply (with a garden sprayer or sponge), then wash off; this procedure is also suitable for the yellow "moustache" on the bow at the end of the season. Then rinse the GRP thoroughly with water and seal.
For stubborn stains, apply some powder to the area, dissolve with water, place a wet sponge on top and leave to work overnight. Hydrochloric acid, citric acid or CIF cleaner also usually help to remove flash rust.
Tips from the expert
Care is required to keep the stainless steel surface clean and therefore rust-free. As the skipper of mega yachts in Florida and the Bahamas, Bernd Altmeier is also responsible for their maintenance. He knows: "After every trip in the Atlantic, however short, the stainless steel has to be properly cleaned of its biggest enemy, salt. And every two months, we polish all the stainless steel parts with Flitz to seal the surface." Further tips from the German skipper: "You shouldn't use any rough agents or cloths, only chamois leather (chamois leather) or cotton cloths are suitable for this, never microfibre cloths." If larger areas need to be freed from surface rust, he also recommends pretreating with oxalic acid. Screws should be replaced in good time before the rust can penetrate the gelcoat in the first place.
But it is not enough just to desalinate the stainless steel parts when washing - the entire boat must be salt-free, otherwise salt will run back onto the metal parts the next time it rains.
This article is part of the special season start. All parts:
- Cast off - how to get the new season off to a smooth start
- Checklist: Fit for the new season - the checklist
- Cleaning, polishing and care
- The best care and cleaning products for boats
- Last-minute tips from the experts at Pantaenius for the start of spring
- Checklist for the boat trailer
- The best tips from the Pantaenius specialist
- Changing the oil in a boat engine - here's how!
- Special pumps - changing oil in no time at all
- To check the cooling circuit
- How to renew the antifouling
- Boat equipment - the more, the better