Vehicle electrical system: Comparison of lead and lithium batteries - Full charge
Leon Schulz
· 14.04.2023
Lead-acid batteries have been on the market for over 150 years. In 1854, Wilhelm Sinsteden was able to produce electricity from lead and sulphuric acid for the first time. His battery was later developed further by the Frenchman Gaston Planté. However, it was not until 27 years later that Henri Tudor managed to build a mass-produced battery. In principle, not much has changed since then. With the introduction of gel-bound acid, the batteries became leak-proof, and finally, in 1972, Gates Rubber developed an acid-soaked glass mat - the "Absorbed Glass Mat" (AGM) battery was born. It can be discharged deeper, can deliver high currents, is less prone to sulphation, does not emit oxyhydrogen and does not leak.
Until recently, such AGM batteries were considered the ultimate in terms of power storage - until lithium batteries came onto the market. The lithium battery works with a completely new technology. The question now is: for which boats are the new batteries suitable, and for whom is the AGM battery, which was highly praised until recently, still suitable?
Lead can be better
One thing in advance: For batteries that are used exclusively for starting the engine, the lead-acid battery, especially the AGM version, is ideal and should not be replaced by lithium.
An AGM battery can deliver the high currents required for starting for a short time, separate ventilation is not necessary and the battery can be relatively small, as it only has to start the engine. As a starter battery, the lead-acid battery has a maximum service life, as it is and remains fully charged at all times - with the exception of the few seconds until the engine starts. The most important argument in favour of the lead-acid battery at this point is that its voltage immediately drops below ten volts as soon as the starter draws current from the battery.
However, this voltage drop in the battery, which is often seen as a disadvantage, is taken into account in the design of the starter motor. With lithium batteries, the voltage does not drop as much under load, so the starter motor receives considerably more power than intended, which it may not be able to withstand for long. For the increasingly diverse and larger power guzzlers on board such as navigation devices, mobile phones, tablets, notebooks, autopilot, refrigerator, lighting or the popular 230-volt consumers such as coffee machines, toasters, kettles and hair dryers, which are operated by inverters, the question of the suitable battery type cannot be answered uniformly. Instead, you should ask yourself the following questions:
- How high is the daily electricity consumption?
- How often should it be charged?
- How fast should it be loaded?
- What should be loaded?
Power guzzler
The comfort features of modern boats require a lot of electricity. Refrigerators and household appliances are the biggest consumers:


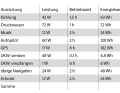



How much energy is needed
When calculating the energy requirement, it is best to start with the power of the respective appliance, which is given in watts (W). Multiplying this by the expected usage time of the appliance gives the daily energy consumption in watt hours (Wh). The values are then divided by the on-board voltage - 12 volts or, in the case of larger vessels, 24 volts - to give the energy required for each appliance in ampere hours (Ah). Adding this up gives the amount of energy that the batteries need to supply each day. The following guide values can be used for orientation: For a day trip, i.e. if you set sail in the morning with fully charged batteries and return to harbour in the afternoon, you will typically have an energy consumption of around 39 ampere hours.
For coastal skippers who use a little more electricity and occasionally anchor or travel at night, around 100 ampere hours are calculated for 24 hours of operation. Long-distance skippers who spend more time on board and use many of the same amenities as on land, including an inverter for 230-volt household appliances, often consume 250 ampere-hours in 24 hours.
How often do you charge?
Another question whose answer depends heavily on the usage behaviour of the boat. For example, if you are moored in a marina every evening, you will most likely always charge using shore power. If you want to anchor from time to time, you may only want to charge every few days. Despite anchoring, many people decide to charge the batteries by engine every day as a routine on board in order to generate hot water for rinsing or showering.
What is used for charging?
Once the battery has lost its energy, it needs to be topped up as quickly as possible. On motorboats, this is done continuously, as the engine is already charging as soon as the boat is disconnected from shore power. Apart from a few short swimming stops, the engine charges practically continuously, unless the crew anchors overnight. As soon as the engine is running again, the charging current is available for the entire journey. The speed of the charging process does not play a major role here; it is sufficient if the batteries are full again the next morning.
The usable capacity
A lead-acid battery should never be fully discharged. How deep a battery can be discharged depends on the design of the lead plates. So-called deep-cycle batteries have thicker lead plates than those designed for engine starting. AGM batteries can also usually be discharged deeper than open, wet lead cells. The expected service life of the battery is heavily dependent on how often and how deeply it is discharged, and especially on how quickly the battery is fully charged again after use. As a general rule, the deeper the battery is discharged, the more often it is deep-discharged and the longer it takes to fully recharge, the shorter its service life. An open lead-acid battery can last for many years if it is almost never discharged and immediately recharged, as the example of the engine start battery shows.
On the other hand, a battery can be destroyed within weeks if, for example, it has been discharged below 50 per cent and remains in this state for a longer period of time. To ensure that the batteries last as long as possible, cautious people never remove more than 50 per cent of the nominal capacity from AGM batteries (state of charge of 50 per cent or SoC 50 for short) and never more than 35 per cent of the capacity from open lead batteries (SoC 75). The disadvantage of this is that the usable energy is at most half of what is stated on the label. In other words, a lot of useless lead has to be driven around.
How long it takes to load
Lead batteries can only be charged to around 75 to 80 per cent at a significant speed. If the battery is fuller than 80 per cent, it can no longer absorb larger currents and the charging speed decreases rapidly. Unfortunately, the range between 80 and 100 per cent is of particular interest on a boat, as the lead-acid battery should be as fully charged as possible to ensure a long service life. However, the maximum charging current is also limited when the charge level is less than 80 per cent. In order not to shorten the service life unnecessarily, charging should not be carried out with currents greater than 20 per cent of the battery capacity. A 100 ampere-hour lead-acid battery should therefore not be charged with more than 20 amperes, which is specified as 0.2 C. The gentler the charging, the longer the service life.
Quickly full again
The charging behaviour is one of the main advantages of lithium batteries:


This is where the lead batteries differ in detail: open lead batteries should be charged particularly slowly, ideally with only 0.1 C, while AGM batteries can be charged with up to 0.3 C. This is also the reason why lead-acid battery chargers have three phases. The first phase is called bulk. This is charged with the highest possible charging current, i.e. with a 100 ampere-hour battery with no more than 20 amperes. The bulk phase lasts until a preset battery voltage is reached; for AGM, this is typically 14.4 volts, which corresponds approximately to SoC 80.
The charger then switches to the absorption phase. Charging now takes place more and more slowly at a constant voltage, as the charging current decreases rapidly. The duration of the absorption phase is specified by the charger and usually lasts many hours. When it has expired at some point, the charger comes to the conclusion that the battery should finally be full. It switches to the third phase, known as the float phase. And its task is to keep the battery at its "feel-good voltage", for example at 13.2 volts.
Some particularly intelligent chargers do not go by time, but interpret a charging current approaching zero as a fully charged battery and then switch to float. Even then, however, a full charge takes a very, very long time.
As so little is charged in the end, many skippers at anchor hardly use more than 30 per cent of their lead batteries when they charge and discharge between 50 and 80 per cent of the capacity. However, this shortens the service life of the lead batteries, which need to be fully charged regularly.
This partial charge is particularly critical for open lead batteries. AGM can withstand the half-discharged state a little longer. However, even AGM batteries should be recharged to 100 per cent every few weeks using shore power or a longer boat trip.
Inside what it says on the tin
With lithium batteries, on the other hand, the specified capacity can be almost fully utilised. Between 80 and 90 per cent of the stored energy can be discharged without damaging the batteries. Only the last ten to 20 per cent is reserved for the battery management system (BMS) integrated in the battery, without which lithium iron phosphate batteries (LiFePO4) should not be used on board.
However, the batteries should not be placed in winter storage with such a low residual capacity, as otherwise the remaining energy could fall below the critical limit. The BMS then switches off completely and recharging is no longer possible without further ado. It is better to charge the battery to 50 to 80 per cent before winter. Some batteries are also equipped with a special winter button that sends the electronics into deep sleep, or the fuse can be removed from the battery so that the BMS computer does not remain switched on during the winter and does not discharge the battery.
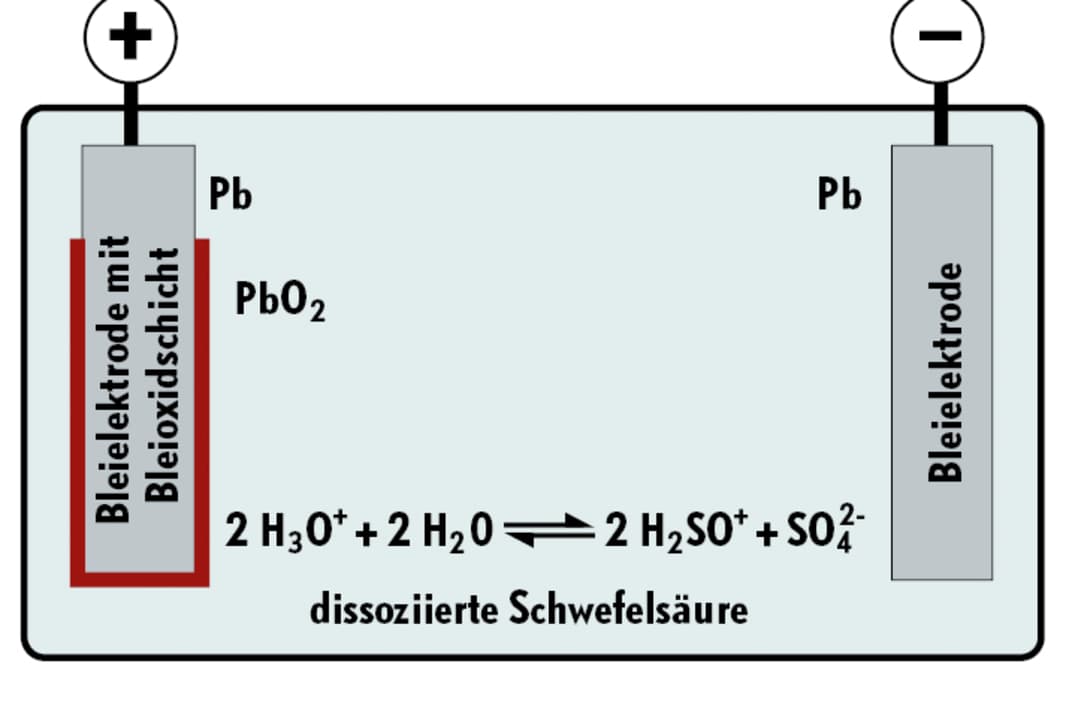
Battery chemistry
Proven technology based on lead-acid or space and weight-saving lithium batteries for fast charging:
In contrast to lead batteries, it does not harm the lithium battery to remain at a low charge level for a longer period of time; on the contrary, it even ages more slowly than when fully charged. Always being connected to shore power, as many lead-acid battery owners are used to, is therefore not recommended with lithium batteries; at the very least, the charger should be switched off.
To set sail with full batteries, it is sufficient to activate the charger one hour before setting sail, as lithium batteries can be charged in a very short time. While lead batteries should be charged with a maximum of 0.3 C, a LiFePO4 battery can be charged with one C without any problems, i.e. a 100 ampere-hour battery with a full 100 amperes of charging current. In addition, this high current flows during practically the entire charging process, which further reduces the charging time.
To fully utilise the advantages, the charger should have a lithium characteristic curve that works with the bulk and float phases. If only a three-phase charger is available, the superfluous absorption phase should be set as short as possible.
Weight
When comparing an AGM battery with 100 ampere hours and a lithium battery of the same size, the difference in weight is immediately noticeable: An AGM battery weighs around 30 kilograms, whereas the lithium battery only weighs around 14 kilograms.
If the mass is set in relation to the usable capacity, the difference is even greater. The day tripper, who has a shore power connection available every evening, can utilise around 50 percent of the battery capacity with lead and weighs 0.6 kilograms per ampere hour. On long voyages, when only between 50 per cent and 80 per cent is charged and discharged during regular anchoring, only 30 per cent of the capacity is used, resulting in a weight of around one kilogram per usable ampere hour.
The situation is different with lithium: As almost 100 per cent of the capacity can be used, the lithium battery weighs just 150 grams per ampere hour.
Price question
The price of lithium batteries puts many boat owners off. While a high-quality AGM battery with 100 ampere hours costs just under 300 euros, lithium batteries of the same size are available for between 1000 and 2000 euros.
To calculate the investment for a new battery, divide the costs by the usable ampere hours. While the investment for an AGM battery, depending on whether 50 or 30 per cent of the capacity is used, amounts to six to ten euros per ampere hour, the lithium battery, at least in the most expensive version, costs twice as much, namely 20 euros per ampere hour.
If the number of cycles is also taken into account, the picture looks more favourable. The service life of the AGM battery is specified in the data sheet as 400 charging and discharging cycles, whereas for lithium it is several thousand cycles. The question of the depth of discharge for which these cycles apply to AGM is justified, but in practice it does not play such a major role, because on most boats the life of a lead-acid battery does not end after the number of cycles has been reached, but already before due to sulphation.
Advantages and disadvantages:




Lead batteries consist of lead plates and sulphuric acid. If the battery remains in a partially charged state for a longer period of time, the lead sulphate crystals grow together to form ever larger clusters, which reduces the capacity of the battery more and more. The deeper the discharge and the longer it takes until the next full charge, the faster the sulphation progresses. AGM batteries are somewhat less sensitive to sulphation than open lead batteries. However, you can try to dissolve the crystals in open batteries by shock charging with increased voltage; some chargers offer special desulphation programmes for this. This process causes oxyhydrogen gas to form and electrolyte is lost, so distilled water must be topped up. Once sulphates have formed in gel or AGM batteries, they cannot be reversed. Desulphation programmes must not be used with these types.
If the service life of lead batteries is assumed to be 400 cycles despite sulphation, each ampere hour costs 1.5 to 2.5 cents, while lithium is the more cost-effective solution at one cent per ampere hour and cycle. Lithium batteries can certainly make economic sense, but usually only pay for themselves after many years.
Charging with the alternator also shortens the charging time and saves fuel - not to mention the reduction in CO2 emissions.
The power system
If lithium batteries are to be used, the entire on-board power concept must be reconsidered. The installation of lithium batteries, for example to shorten the charging time, will be disappointing if the alternator is not designed to continuously supply high charging currents. Similarly, the running time of the 230-volt diesel generator, however large, will not be shortened if it is connected to a charger that is far below the possible charging capacity of the battery.
It can therefore make a lot of sense to install a second high-performance alternator on the motor. It should have a charging characteristic for lithium batteries and deliver high amperages even when warm. Side effect: If one of the charging systems is defective, the still-functioning alternator can charge the engine and consumer batteries by switching over or using temporary cabling.
Finally, all cables, fuses and contacts on board should be checked and replaced if necessary. Because if high charging currents suddenly flow, the entire system must be well harmonised.